A site master file or SMF is a document in the which provides information about the production and control of. The document is created by a manufacturer. About [ ] A site master file is a document prepared by the manufacturer containing specific and factual information about the production and/or control of pharmaceutical manufacturing operations carried out at the named site and any closely integrated operations at adjacent and nearby buildings. If only part of a pharmaceutical operation is carried out on the site, the site master file need describe only those operations, e.g., analysis, packaging.
See also [ ] • • References [ ].
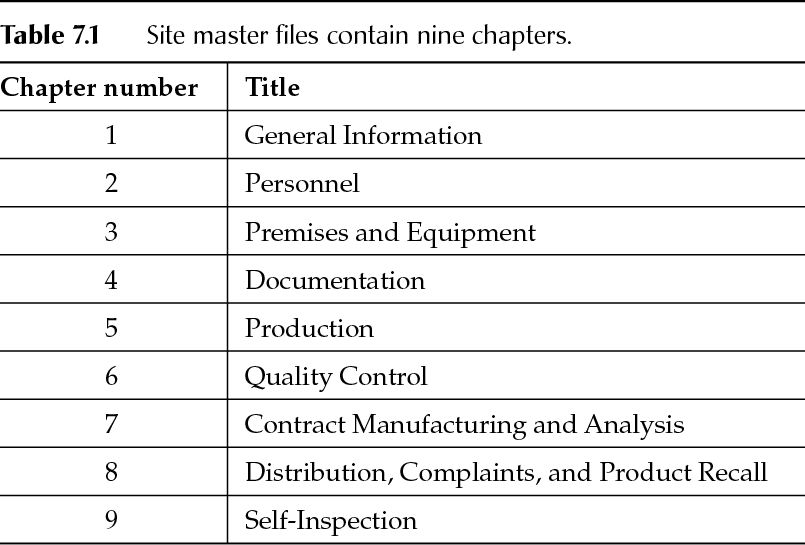
A thorough review of the elements every Site Master File must include And much more. Your guide, Cornelia Wawretschek, is author of a chapter in the GMP Compliance Adviser, Good Manufacturing Practices and Implementation, from which The Drug Manufacturer’s Guide to Site Master Files is adapted. Who guidelines for drafting a site master file. Guidelines for drafting a site master file (smf). Heroes Season 2 English Subtitles Download on this page. (gmp) information about.